Development of Dihydrodibenzooxepine Peroxisome Proliferator-Activated Receptor (PPAR) Gamma Ligands of a Novel Binding Mode as Anticancer Agents: Effective Mimicry of Chiral Structures by Olefinic E/ Z-Isomers.
Yamamoto, K., Tamura, T., Henmi, K., Kuboyama, T., Yanagisawa, A., Matsubara, M., Takahashi, Y., Suzuki, M., Saito, J.I., Ueno, K., Shuto, S.(2018) J Med Chem 61: 10067-10083
- PubMed: 30351933
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01200
- Primary Citation of Related Structures:
6AD9 - PubMed Abstract:
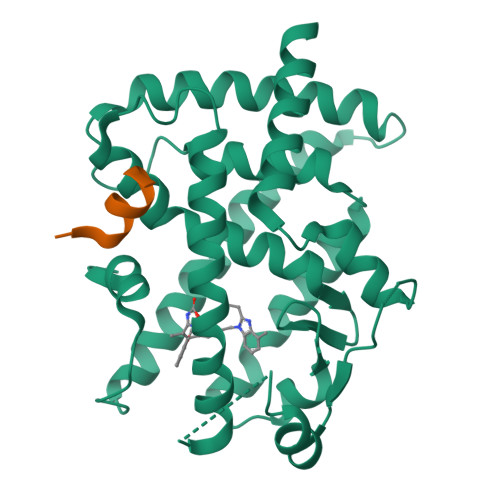
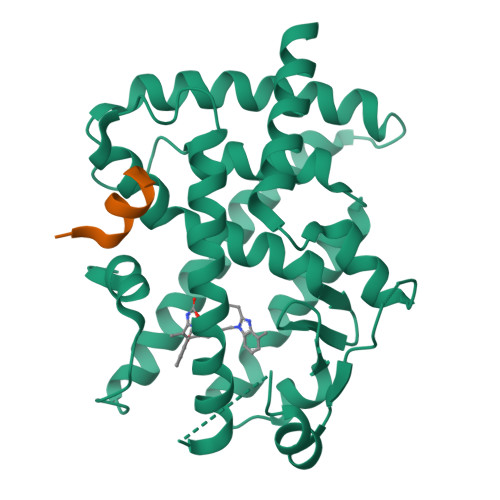
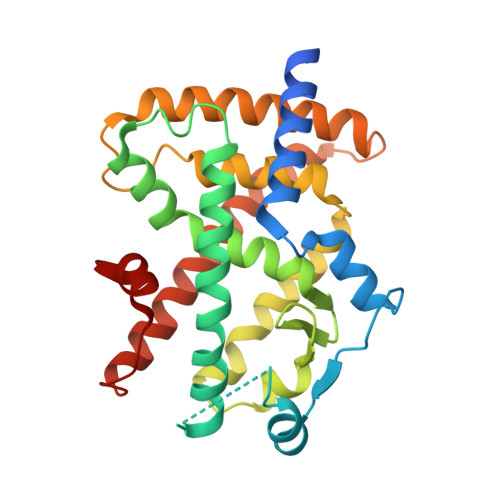
A novel class of PPARγ ligand 1 (EC 50 = 197 nM) with a dibenzoazepin scaffold was identified through high-throughput screening campaign. To avoid the synthetically troublesome chiral center of 1, its conformational analysis using the MacroModel was conducted, focusing on conformational flip of the tricyclic ring and the conformational restriction by the methyl group at the chiral center. On the basis of this analysis, scaffold hopping of dibenzoazepine into dibenzo[ b, e]oxepine by replacing the chiral structures with the corresponding olefinic E/ Z isomers was performed. Consequently, dibenzo[ b, e]oxepine scaffold 9 was developed showing extremely potent PPARγ reporter activity (EC 50 = 2.4 nM, efficacy = 9.5%) as well as differentiation-inducing activity against a gastric cancer cell line MKN-45 that was more potent than any other well-known PPARγ agonists in vitro (94% at 30 nM). The X-ray crystal structure analysis of 9 complexed with PPARγ showed that it had a unique binding mode to PPARγ ligand-binding domain that differed from that of any other PPARγ agonists identified thus far.
Organizational Affiliation:
Fuji Research Park, R&D Division, Kyowa Hakko Kirin , 1188 , Shimotogari, Nagaizumi-cho, Sunto-gun, Shiuoka , Japan.